Researchers at Tokyo University of Science (TUS) have developed a new electrolyte material that improves the conductivity of magnesium ions at room temperature, paving the way for the next step in the development of magnesium ion (Mg2+) batteries. According to researchers, Mg2+ batteries, considered a cheaper alternative to lithium-ion batteries, have faced major obstacles due to the poor conductivity of magnesium ions in solids at room temperature.
"The lithium-ion battery has an advantage in gravimetric energy density, so it is suitable for mobile use (such as a phone)," said Masaaki Sadakiyo, Ph.D., junior associate professor, Division I, Faculty of Life Sciences, Department of Applied Chemistry. in TUS But lithium (Li) is a rare element, he said.
"On the other hand, the Mg2+ battery has an advantage in volumetric energy density and cost (i.e., less use of a rare element), which will be beneficial in stationary applications (eg, energy storage for renewable sources)," he added. "Given that lithium is a limited resource on Earth, large-scale energy storage in the future world must be replaced by other batteries such as Mg2+."
Magnesium is a promising solid-state battery material due to its abundance, and Mg2+-based power devices have high energy density, high safety and low cost, researchers say. However, the widespread use of Mg2+ has been limited by its poor conductivity in solids at room temperature, they reported: “Mg2+ has poor solid-state conductivity because the divalent positive ions (2+) experience a strong interaction with neighboring negative ions in the solid. crystal, preventing their migration through the material."
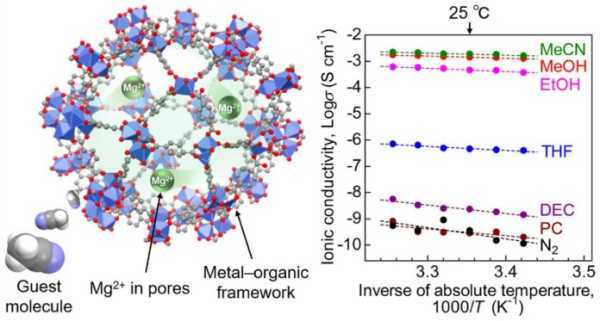
Researchers at TUS believe they have solved the chemical confinement problem with a metal-organic framework (MOF)-based Mg2+ conductor with superionic conductivity at room temperature. They reported that the Mg2+ electrolyte achieved a superconductivity of 1.9 × 10–3 Cm–1, which is the threshold for practical application in solid-state batteries.
The researchers shared their findings in a study published in the Journal of the American Chemical Society. A key result shows that the conductivity is the highest to date for a crystalline Mg2+ solid, breaking a decades-long barrier.
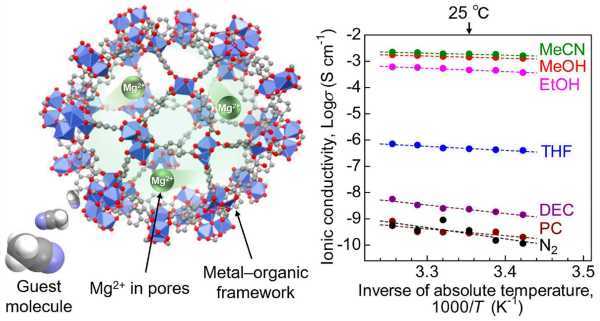
Sadakiyo, who led the research on "A Novel Lithium-Free Magnesium Superion Conductor to a Solid State." -State Batteries,” described materials used as MOFs that have a highly porous crystalline structure that enables efficient ion migration.
The researchers introduced a "guest molecule", acetonitrile, into the pores of the MOF, which accelerated the conduction of Mg2+.
Conductor Mg2+, consisting of a metal-organic base. Click to enlarge image. (Source: Masaaki Sadakiyo, Tokyo University of Science)
"Our paper is related to the development of an electrolyte for solid-state batteries (ie, the Mg2+ conductor)," Sadakiyo said. "We prepared a new Mg2+ conductor and clarified its ion-conducting mechanism. We explained that Mg2+ contained in the pores of a certain solid material (i.e. MOF) migrates efficiently under certain organic vapors, and that the resulting conductivity of Mg2+ is high enough to be used for a battery.”
The team used a MOF called MIL-101 as a scaffold, encapsulating Mg2+ ions in its nanopores. This created a MOF-based electrolyte where Mg2+ was loosely packed, allowing the migration of divalent Mg2+ ions. Then, to improve ionic conductivity, acetonitrile vapor was introduced into the electrolyte. The samples were subjected to an AC impedance test to measure ionic conductivity.
Other measurements and tests have shown that acetonitrile molecules adsorbed in the framework ensure efficient migration of Mg2+ ions through the solid electrolyte body. This demonstrated that the MOF-based Mg2+ conductor is a suitable material for batteries.
Sadakiyo believes this breakthrough brings the industry one step closer to a commercial Mg2+ battery, although more work is needed in other areas. "We believe our findings contribute to the realization of a Mg2+ battery from an electrolyte perspective," he said. "However, as you mentioned, there are many other challenges involved in making a practical Mg2+ battery, not just for the electrolyte materials, but for the electrode materials as well."
The research team plans to apply this material to a "real" battery in collaboration with another lab. Sadakiyo believes that a special license is not needed for commercial use because they have not received a patent and the work has already been published. "However, at this point, we believe there are many challenges that need to be addressed by additional researchers to achieve real-world use of the battery."
One of the next steps for the team includes creating "other new materials that exhibit higher Mg2+ conductivity with a higher Mg2+ transport number with lower organic vapor pressures," Sadakiyo said. The team is also "interested in covering the fundamental science of the conductivity of multivalent ions (such as Mg2+) in the solid state."
According to Sadakiyo, we won't see commercial Mg2+ batteries available on the market for at least 10 to 20 years.
In addition to Sadakiyo, the research team includes Yuto Yoshida, also of TUS; Professor Teppei Yamada from the University of Tokyo; and Associate Professor Takashi Toyao and Professor Ken-ichi Shimizu from Hokkaido University.
Source: electronicproducts.com










